Nov 11, 2019 in Drug Delivery, Press Releases
XPhyto Therapeutics oral and transdermal Cannabis Delivery and Dosage Systems
Vancouver, Canada (November 11, 2019) – XPhyto Therapeutics Corp. (CSE:XPHY; FSE:4XT) (“XPhyto” or the “Company”) is pleased to provide an update on the Company’s thin film cannabis delivery and dosage systems in development by XPhyto’s wholly owned German subsidiary, Vektor Pharma TF GmbH (“Vektor”). Vektor is a narcotics manufacturer, importer, and researcher located near Munich, Germany.
For over a decade, Vektor’s founder and managing director, Professor Thomas Beckert, has been a leader in the development, testing and manufacture of thin film narcotics delivery systems, primarily transdermal patches and sublingual (oral) strips for the clinical management of pain. In addition to Vektor’s expertise working with conventional pain narcotics such as Fentanyl, Hydromorphone, and Oxycodone, recent work has focused on the development of efficient cannabis delivery and dosage systems.
As the cannabis industry evolves, the opportunity to produce a well-defined dosage form has emerged as a major opportunity for XPhyto. High bioavailability with controlled, reproducible, and constant delivery is paramount. Results from initial studies using Vektor’s formulation and oral delivery system for CBD have been extremely encouraging, demonstrating significant potential timing and efficiency advantages over other common oral methods (preliminary results in Figure 1 below).
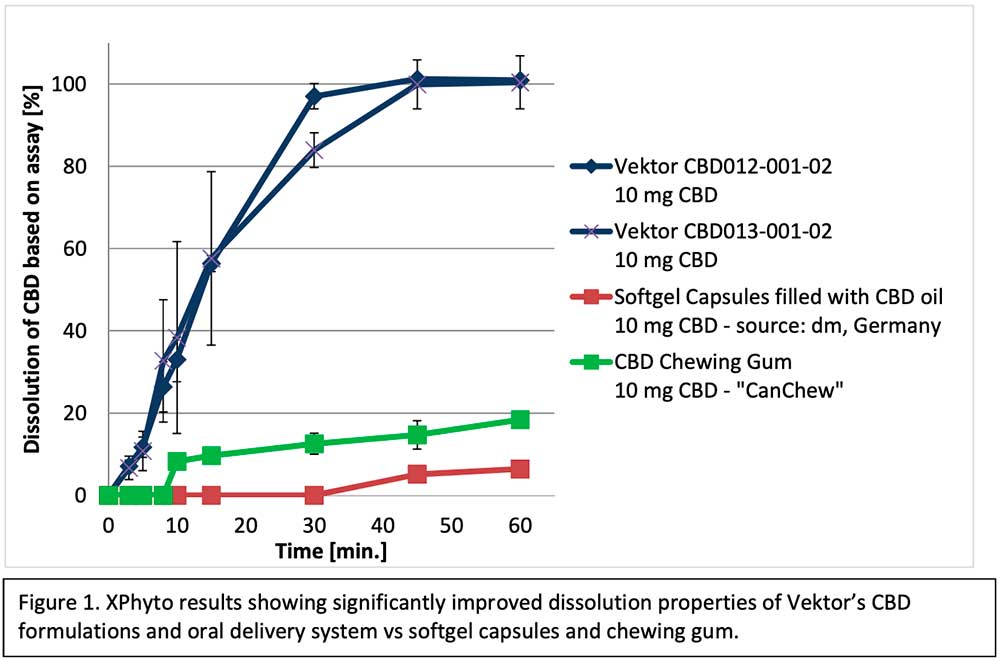
Strong scientific data supporting XPhyto’s non-inhalable cannabis delivery systems is significant in light of recent reports concerning e-cigarette and vape-related illnesses in North America. In the past 30 days, both the US Food & Drug Administration (FDA) and the US Centers for Disease Control & Prevention (CDC) have issued public statements warning consumers about lethal potential side effects that may be associated with e-cigarettes and vaping products. XPhyto and Vektor are prioritizing non-inhalable cannabis development programs for clinical advancement.
In addition to the global medical market, XPhyto is exploring near-term commercial opportunities for thin film consumer products in legal jurisdictions, including Canada.